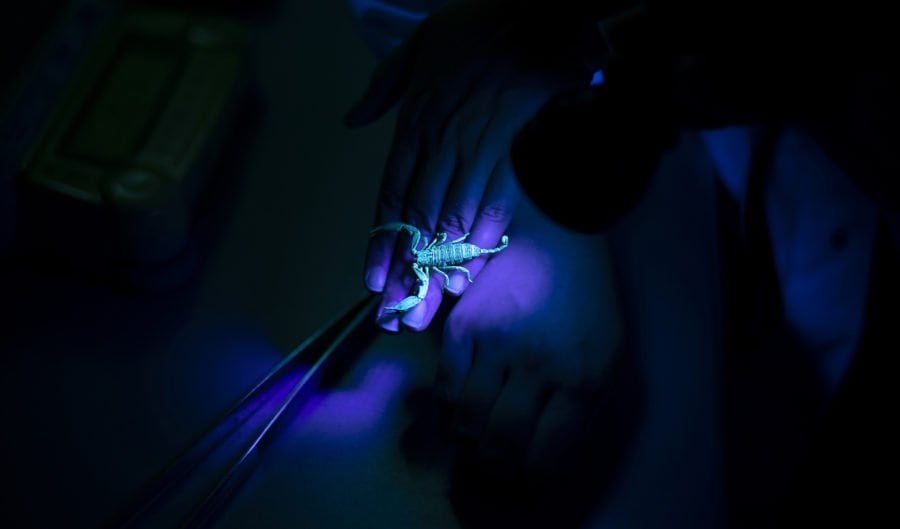
Man deliberately bitten by snakes helps create new antivenom

One man’s habit of injecting himself with the venom of the world’s deadliest snakes has led to the creation of a new antivenom.
The team that created it say they could be on a path to developing a universal antivenom – an important goal, given it’s estimated that more than 5 million people are bitten by venomous snakes every year, and tens of thousands of those people die.
In Australia, snake-bite deaths are rare. But some of our deadliest snakes feature prominently in the story of how this new antivenom was made.
How the antivenom was created
American snake enthusiast Tim Friede began injecting himself with snake venom in 2001, and kept doing so for 17 years, reportedly recording 856 self-injections and deliberate snake bites.
“It became a lifestyle for me,” Friede told American television network CNN this week. “It’s an addiction in [that], as far as I know, I’m helping humanity.”
The videos Friede posted online of himself being bitten by snakes attracted global interest, including from antivenom researchers.
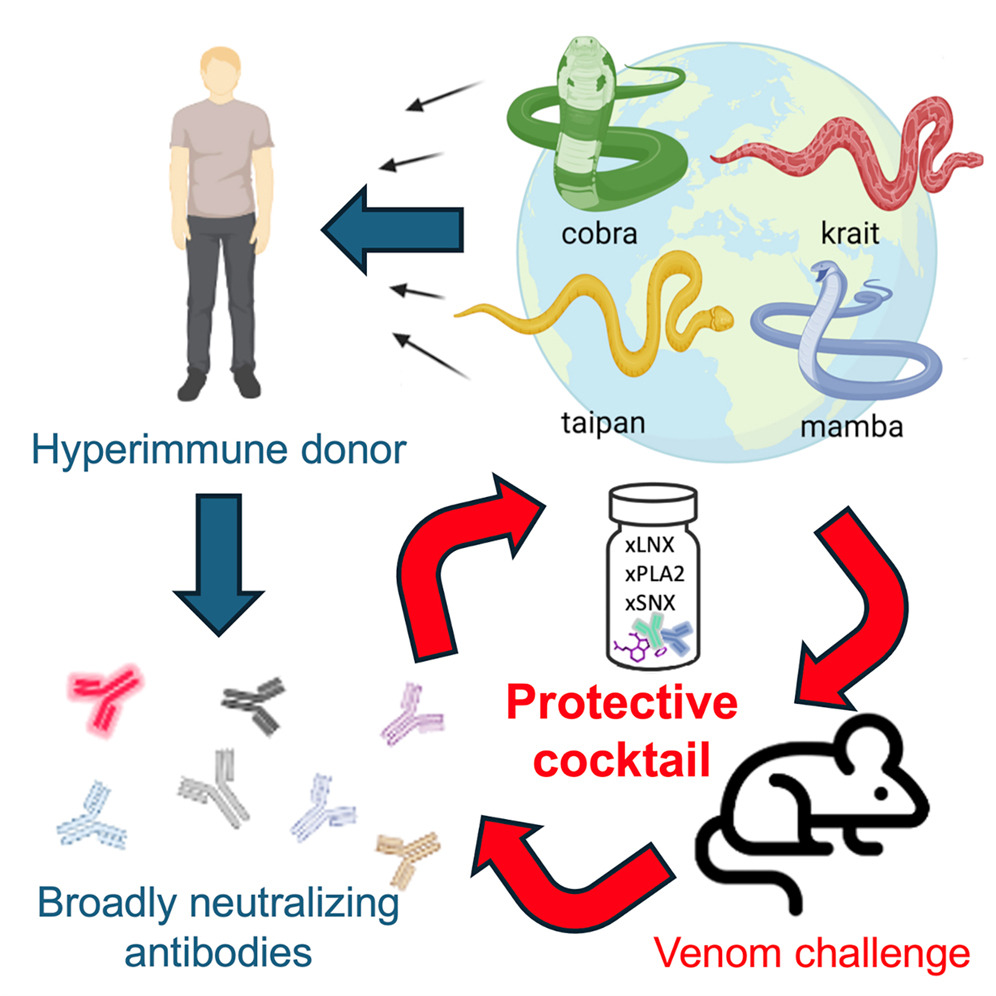
Antivenoms have traditionally been developed with antibodies collected from large animals such as horses. But a team of scientists from the USA’s Columbia University and vaccination research company Centivax saw potential for Friede’s own antibodies, as a survivor of hundreds of snake bites and self-injections, to form the basis of a new antivenom.
The research team mixed a ‘cocktail’ of venom neurotoxins, toxin inhibitors and Friede’s antibodies to develop their new antivenom. They say they have created the most broadly effective antivenom to date and have published details of their research in the journal Cell.
“What was exciting about the donor [Friede] was his unique immune history,” said Jacob Glanville, the CEO of Centivax, where Friede also now works as director of herpetology.
“Not only did he potentially create these broadly neutralising antibodies, [but] in this case it could give rise to a broad-spectrum or universal antivenom.”
The Australian connection
Friede’s fascination with venomous snakes extends to many of Australia’s deadliest.
“Tim has records of self-immunising with multiple Australian snakes, including the inland taipan, coastal taipan, eastern brown snake and tiger snake,” Glanville told Australian Geographic.
“Notably, his antibodies provided mice complete protection to mulga-snake venom, and partial protection to common death adder venom, despite not being part of his self-immunisation history.”



The research team believe their new antivenom could be particularly effective in Australia because Australia’s venomous snakes are all elapids – that is, snakes with permanently erect fangs at the front of their mouth.
The new vaccine has proven effective against elapid venom, but less so for the venom of vipers, a family of fang-folding venomous snakes found in much of the world but not in Australia.
“Australia is unusual, in that its venomous snakes are comprised of only the elapids, the neurotoxic snakes,” said Columbia University medical sciences professor Dr Peter Kwong, who was part of the research team. “But for this reason, an antivenom that works mainly for elapids can be tested in Australia.”
Australian researchers remain cautious
Australian snake venom experts said the new research is fascinating, but they’re remaining cautious about the antivenom’s effectiveness in Australia.
Dr Timothy Jackson, the co-head of the Australian Venom Research Unit at The University of Melbourne, told Australian Geographic he’s not convinced the new antivenom will be as useful in Australia as the research team hopes.
“It is cool; I’m not saying the science isn’t good. But it does miss certain key points,” Jackson said.
“I would say they are on the pathway to a very broad-spectrum antivenom, and that’s a good thing. But if we were referring just to Australia, then there’s really no need for such a product. The [existing] Australian antivenoms are notably broad spectrum.
“The situation is very different elsewhere in the world though, so there is a great need for better, more broad-spectrum improvements on antivenoms for places like India, sub-Saharan Africa, South and Southeast Asia – although we also need to address issues with health infrastructure in these places.”


Jackson said he remains cautious about the new antivenom’s value in Australia because the way the researchers studied how snake venom kills rodents in a laboratory setting is very different from the way snake venom affects animals and humans outside the lab.
“I don’t want to take away from the quality of the science, because I think they have actually done a really good and comprehensive study on the immunological side of things,” Jackson said.
“But snake venoms attack the bodies of target animals in lots of different ways simultaneously. In fact, lethality is a kind of secondary side effect of venoms in general. Snake venoms are either there to subdue target animals or to deter potential predators.
“It’s a very broad, general characterisation that elapids go heavy on neurotoxins, but they do other things too. This would be a really good product, I suspect, against a lot of elapids that are predominately neurotoxic, but in Australia that’s not going to cover our most clinically relevant snakes, especially taipans and brown snakes. It would probably be really good for a death-adder bite, but not necessarily for a brown-snake bite.”
Aussie vets could conduct field tests
While the new antivenom has so far only been tested on mice, Australia could play host to the next round of testing on real-world snake-bite victims. The antivenom’s developers said they’ve been in contact with Australian emergency vets about testing it on their patients.
“Dogs bitten by snakes provide an opportunity to test the effectiveness of the cocktail,” said Dr Kwong.
“Because it takes an hour or more for most snake-bitten dogs to die, one can ‘test’ the antivenom cocktail and still have a window to provide the current standard of care if the cocktail is not effective in 15-20 minutes.”
What now?
As for the antivenom’s potential use on humans? The research team said that’s years away, with extensive clinical testing and manufacturing still to come.
For now, Glanville had this parting advice for anyone tempted to conduct their own version of Tim Friede’s research: “Venom is dangerous. Please don’t do this at home.”
Jackson also emphasised the danger Friede exposed himself to.
“Having a human in the loop and getting your broad-spectrum antibodies from a human makes for a good story but is unnecessary. He has done something really dangerous, and it’s his personal choice to do that, but I think it is risky to paint him as a hero. You could use any mammal to generate those antibodies. And we can pretty much do it without any animals in the loop at this point, so we are moving in a more ethical direction.”